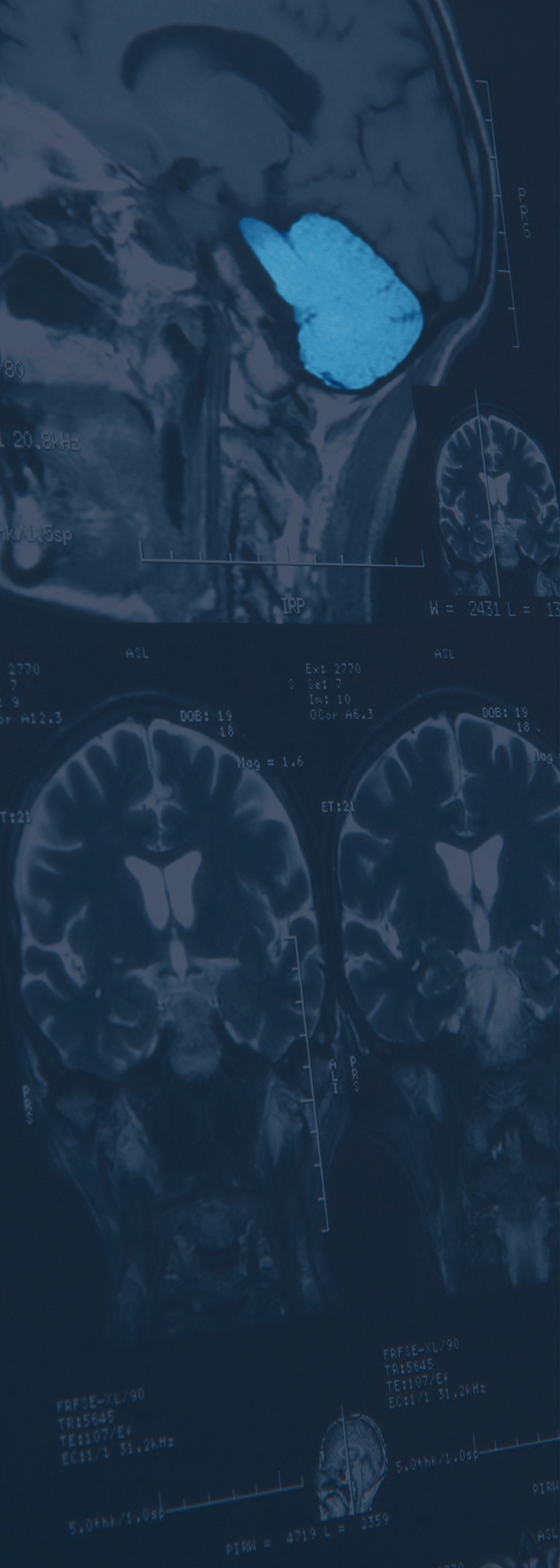
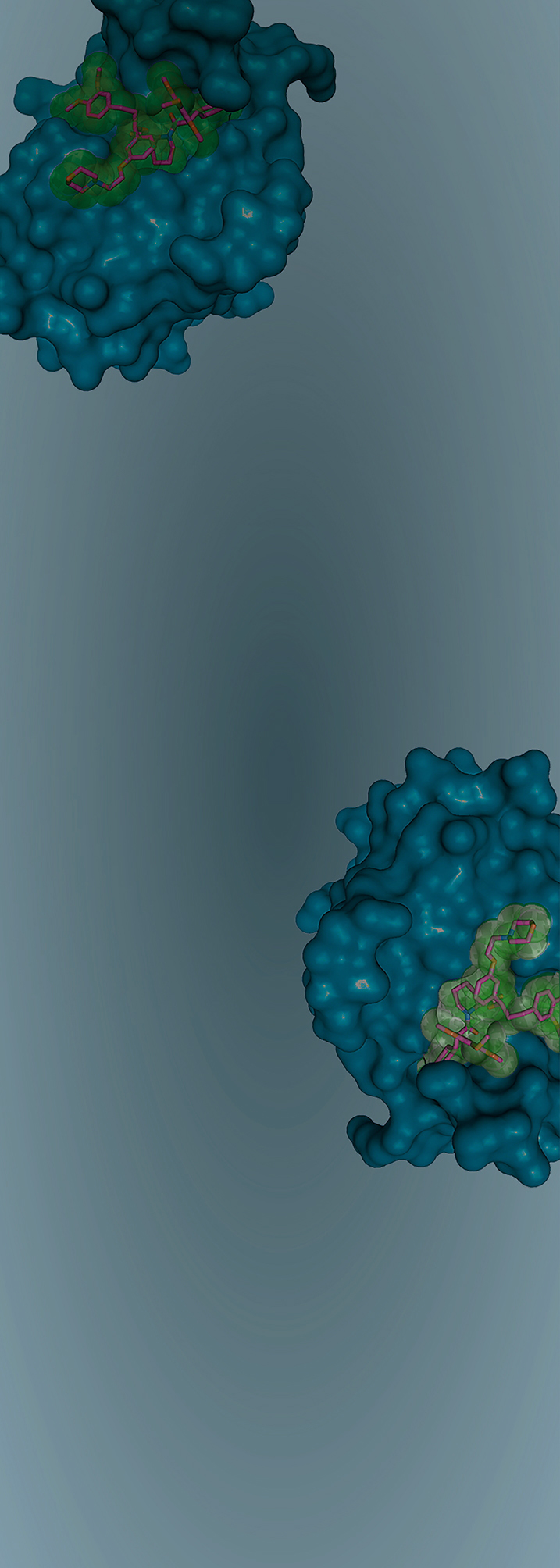
Basic research broadens knowledge. It is only when components and functions of an organ, tissue or cell group are known and understood that approaches for an effective therapy with minimal side-effects can be developed.
Whenever possible, results are obtained using alternative methods to animal experiments. However, non-invasive procedures have mostly low resolution. Thus, the complex interactions of nerve cells with each other and their surroundings, as well as their influence on behavior can only be clarified with the aid of specially bred experimental animals. New methods and improved knowledge allow us today to gain important insights also from studies on flies, for example. No research at the Institutes is done on dogs, cats or monkeys.
In the 1960s, Albert Herz and his team explored the function and mechanisms of action of opium and opioids in mice and rats. The research team identified previously unknown opioids and found out where and how opioids work in the brain. They also showed how the investigated processes are influenced by drugs, stress and pain. The findings of these studies have become part of modern pain therapy (morphine), addiction therapy (methadone) and serve to assess the addiction potential of novel painkillers.
Towards the end of the 1980s, Werner Risau, Axel Ullrich and colleagues at the Max Planck Institute for Biochemistry investigated growth factors that promote blood vessel formation. They found that small tumors produce a factor that stimulates the growth of blood vessels and thus promotes tumor growth. In the 1990s, the necessary animal experiments were carried out on mice in the USA. The end result was a highly effective cancer treatment (SUTENT®), launched in 2006.
Hartmut Wekerle and his team investigate the fundamental causes of multiple sclerosis (MS), the most common autoimmune disease of the central nervous system. In the 1980s, they developed a rat model in which auto-aggressive T cells produce a MS-like disease. With the help of this animal model, Californian researchers were able to further elucidate mechanisms of the disease. This led to the approval of TYSABRI® in 2006, currently the most effective drug for relapsed MS.